Ying Xu, Peikun Zhang, Xiaoyu Xuan, Minmin Xue, Zhuhua Zhang, Wanlin Guo, Boris I Yakobson
The Journal of Physical Chemistry Letters, 2022, 13, 4, 1107–1113
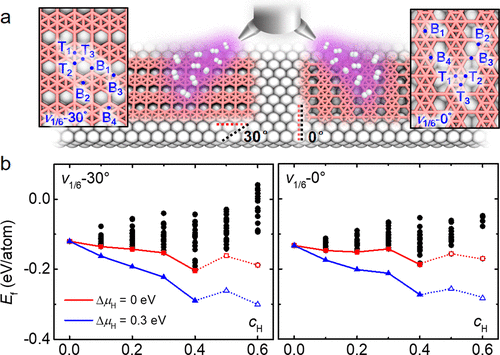
Abstract: Hydrogenated borophenes-borophanes-have recently been synthesized as a new platform for studying low-dimensional borides, but most of their lattice structures remain unknown. Here, we determine the structures of borophane polymorphs on Ag(111) by performing extensive structural search using the cluster expansion method augmented with first-principles calculations. Our results reveal rich borophane polymorphs whose stability depends on hydrogen pressure. At relatively low hydrogen pressures, borophane structures with rhombic patterns of two-center–two-electron B–H bonds are energetically preferred, in excellent agreement with two experimentally observed phases. In a wider range of hydrogen pressures, the structure with a combination of two-center–two-electron B–H and three-center–two-electron B–H–B bonds is a deep global minimum, rationalizing its experimental prevalence. For all these borophane polymorphs, their hydrogen “skin” raises the energy barriers for oxidation above 1.1 eV, while their work functions can be reduced by more than 0.5 eV through varying the hydrogen coverage.