Abstract:
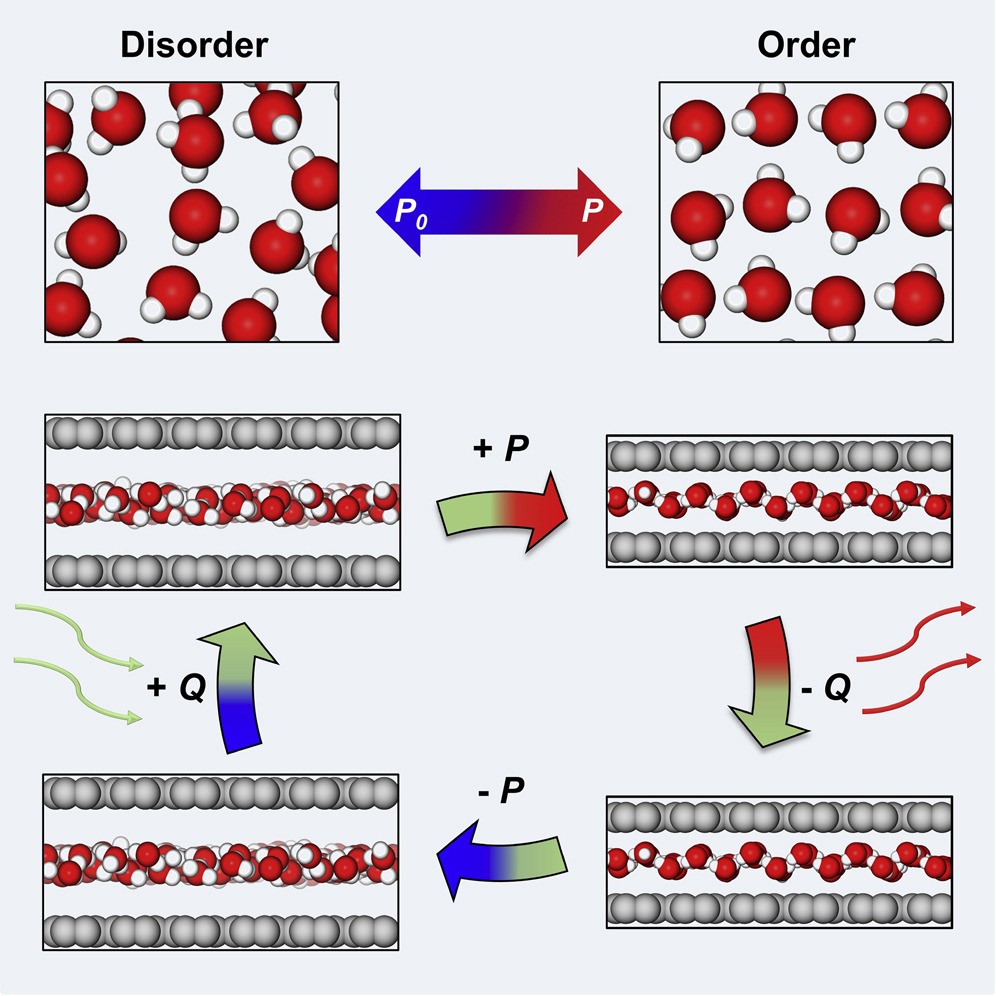
Using water as a refrigerant for innovative cycle-cooling technology is desirable, since water not only is economically and environmentally unrivaled, but also owns a latent heat that is 16-fold that of commercial halohydrocarbon. However, water-based refrigeration has been limited by a phase transition point far from room temperature and intractable volume change upon vaporization. Here, we use a synergy between nanoconfinement and mechanical pressures in regulating the phase transition of water and report a giant mechanocaloric effect in nanoconfined water by thermodynamic analysis and atomistic calculations. The estimated isothermal entropy changes are as high as 1,066 J kg−1 K−1 and adiabatic temperature changes can reach tens of kelvins near room temperature for water confined in slit nanopores. Practical utility of this effect is supported by the robustly high refrigerant performance of water-filled nanoporous carbon matrices. This effect is potentially extendable to other polar liquids and opens new prospects for liquid-state caloric effects.
--校内链接--
--校外链接--

微信公众号