Yan Chen, Zuoqing Liu, Bin Chen, Wei-Hsiang Huang, Zheng Tang, Mingkai Xu, Min-Hsin Yeh, Chih-Wen Pao,
Mingjie Pu*, Guangming Yang, Yufeng Guo, Zhiwei Hu, Yinlong Zhu
Adv. Energy Mater.(IF:24.4),First published:07 August 2025
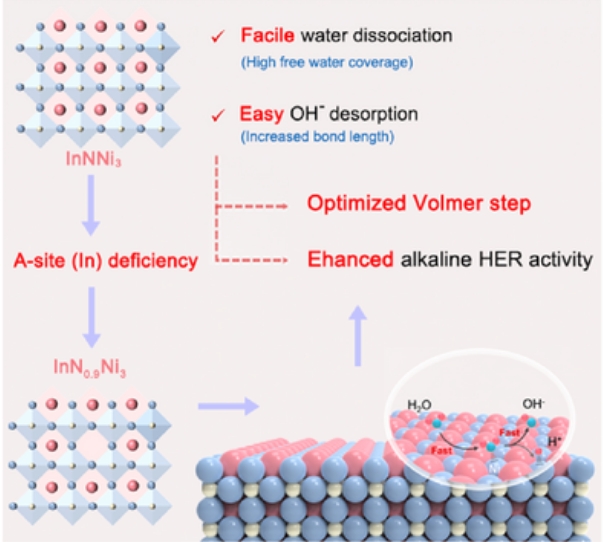
The slow kinetics of alkaline hydrogen evolution reaction (HER) leads to large activity gap between acidic and alkaline electrolytes mainly owing to the extra sluggish Volmer step involving water dissociation and OH− desorption. Herein, a facile and effective strategy is reported to regulate the electronic structures and interfacial water behavior through introducing A-site cation deficiency in antiperovskites for jointly accelerating the Volmer reaction and thereby improving the alkaline HER performance. As a proof-of-concept, the In-deficient In0.9NNi3 antiperovskite shows significantly enhanced HER activity than stoichiometric counterpart and outperforms various state-of-the-art non-noble metal electrocatalysts ever reported. More importantly, when applied in a practical anion exchange membrane water electrolyzer (AEMWE) device, the In0.9NNi3-based cathode attains an industrial current density of 1 A cm−2 at a low voltage of 1.92 V and steadily operates up to 100 h, superior to the commercial Raney Ni catalyst. By combined theoretical studies and comprehensive experiments, the enhanced HER performance of In0.9NNi3 is mainly originated from the Ni site-expedited Volmer step, which includes boosted water dissociation by promoting the free water adsorption and spontaneous OH− desorption due to elongated Ni─N bonds. This work provides a significant inspiration to design high-performance alkaline HER electrocatalysts via defect chemistry.
https://doi.org/10.1002/aenm.202503319
--校内链接--
--校外链接--

微信公众号